Grant for Greenville med device firm is a first in Eastern NC
GREENVILLE – RFPi, a Greenville medical device company, has landed the first Phase II Small Business Technology Transfer grant ever awarded by the National Institutes of Health in Eastern North Carolina or with East Carolina University as the academic research partner.
The company will use the $1.49 million grant from the National Heart, Lung, and Blood Institute to develop its non-invasive proprietary Multi-spectral Physiologic Visualization (MSPV) platform imaging technology for use with patients suffering from peripheral artery disease. This will include building and testing prototypes with animal models and conducting a clinical evaluation to document safety and efficacy. Following this step, RFPi plans to apply for a subsequent award to fund clinical trials necessary to bring it to market.
RFPi received FDA clearance earlier this year to begin marketing its first form factor of MSPV, called iCertainty, for use in open surgery procedures. iCertainty provides real-time analysis of blood flow and perfusion (the rate at which blood is delivered to tissue, such as through a capillary bed, measured in volume of blood per unit of time per unit of tissue mass) without disruption of the surgery or use of any dyes, contrast or even touching the patient. RFPi videos on the company website show how MSPV and the iCertainty application work.
Target uses of the imaging technology, although it has applications across many surgical subspecialties, include in coronary artery bypass graft or bowel resection surgeries where established blood flow and perfusion are critical to their success, said Bruce Ferguson, M.D., RFPi’s chief medical officer and one of the inventors of the technology. Ferguson is a retired cardiothoracic surgeon and was professor and the inaugural chairman of the Department of Cardiovascular Sciences at ECU’s Brody School of Medicine.

Bruce Ferguson, M.D., RFPi’s chief medical officer
His team addressed the problem that providers cannot recognize inadequate blood flow and/or perfusion in tissues using only visible light illumination. MSPV quantifies blood flow distribution (flow and perfusion) in real time. For the provider, the process of getting this information cannot interrupt the surgical procedure or clinic evaluation. For the patient, getting this information should not require injection of dyes and should not increase risk (for example, need ionizing radiation). The new information should help providers make better clinical decisions at the time of the procedure or clinic encounter.
The MPSV solution, unlike other competing technologies, is non-invasive, non-contact, non-ionizing, safe for patients and providers, and provides blood flow distribution in real time. Identifying compromised tissues in real time means the compromise can be fixed in real time.
Complications of inadequate blood flow or perfusion can occur in up to 15% of patients in major surgeries; reducing or eliminating these complications with MSPV imaging would avoid re-operations, additional patient suffering, delayed recovery and additional costs. RFPi believes that in many areas of clinical medicine MSPV’s ability to identify normal vs. abnormal blood flow distribution will improve patient outcomes, healthcare quality, and healthcare value.
“He [Ferguson] is a perfect example, to me, of a clinician who has identified a problem and created a solution to address that problem,” said Greta Brunet, M.B.A., PA-C, senior director of investments and co-leader of the Clinician Innovation Initiative (CII) at the North Carolina Biotechnology Center and a certified and former practicing physician assistant with surgical experience.

RFPI
NCBiotech inaugurated the CII in summer of 2018 to encourage innovation and entrepreneurship by clinicians that will improve patient care. RFPi is in Brunet’s Biotech Center investment portfolio. NCBiotech awarded a $250,000 Small Business Research Loan to the company in 2017 to help develop a clinic-based prototype of the MPSV technology solution.
NIH also recently awarded RFPi a $225,000 Small Business Innovation Research grant for the company to develop an MSPV-based portable, simple-to-use monitoring-type device. Designed for elderly patients discharged following surgical procedures requiring general anesthesia, the device will monitor their cardiovascular status and other measures of well-being, such as peripheral oxygen saturation, from the time of hospital discharge until their first post-surgical checkup, said Ferguson.
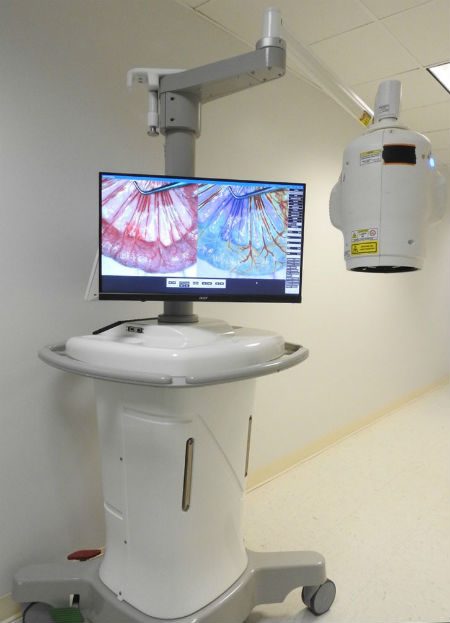
The iCertainty device. (RFPI photo)
This will enable patients and their caregivers to more clearly identify when these measures are variations within the expected range and when they require medical attention. Not only will these data enable clinicians to address post-surgical anomalies more quickly and effectively, but it also will give peace of mind to patients and caregivers when all is well, and allow them to remain at home during recovery without extra clinical visits based on the uncertainty of vague symptoms.
RFPi closed on its Series A round of financing in May 2017. It now has an open round of Series B financing to raise funds for commercializing the FDA-approved iCertainty device and market it to medical centers and their surgical teams.
“This is really gratifying to watch this local company leverage additional funding to develop amazing technology that’s needed to improve clinical outcomes,” said Mark Phillips, NCBiotech vice president of statewide operations and executive director of the Eastern Region Office.
“This region’s global life science stature has been transformed by pharmaceutical manufacturing. We’re delighted that we’re adding breakthroughs in advanced medical technologies such as these to that mix.”

