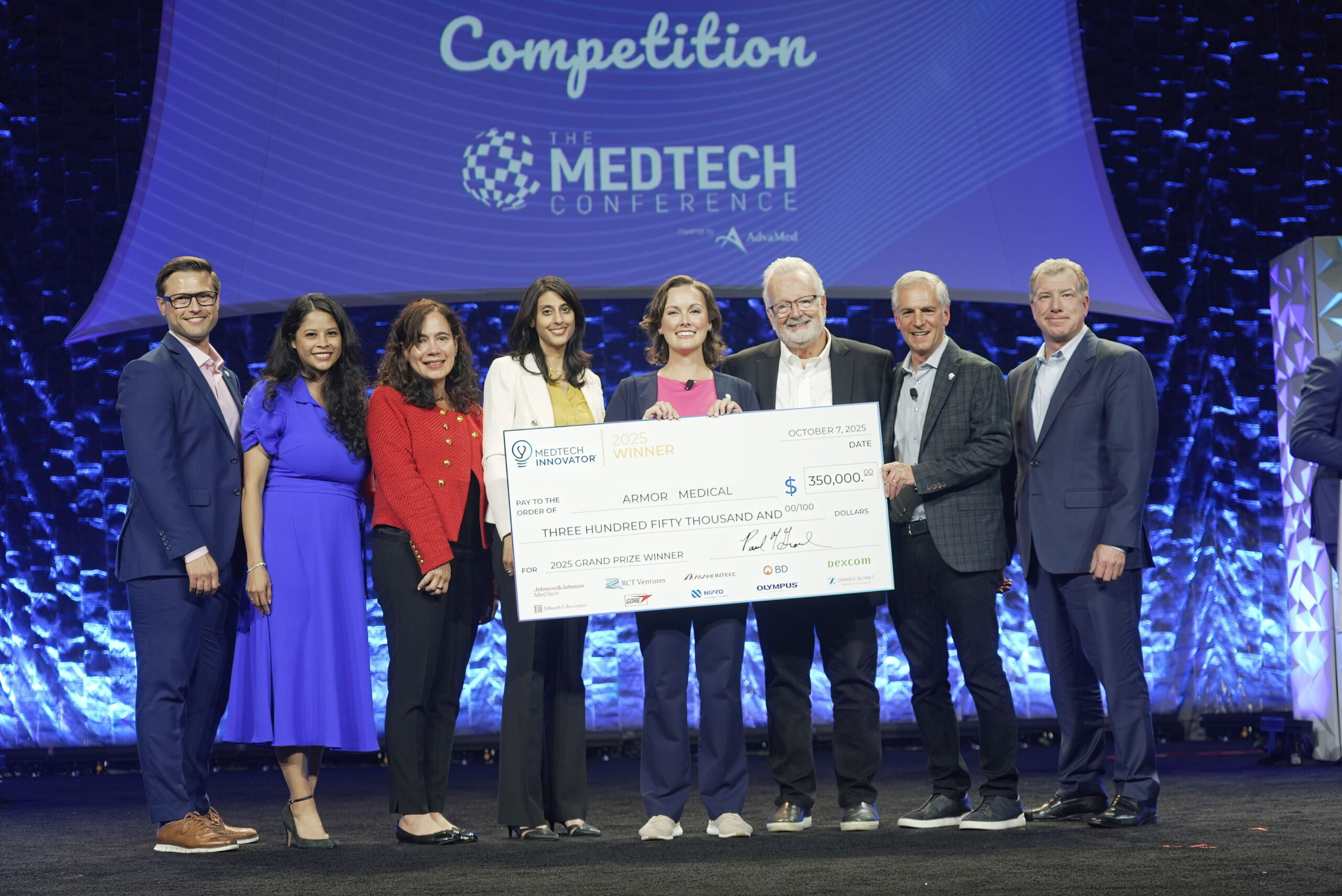
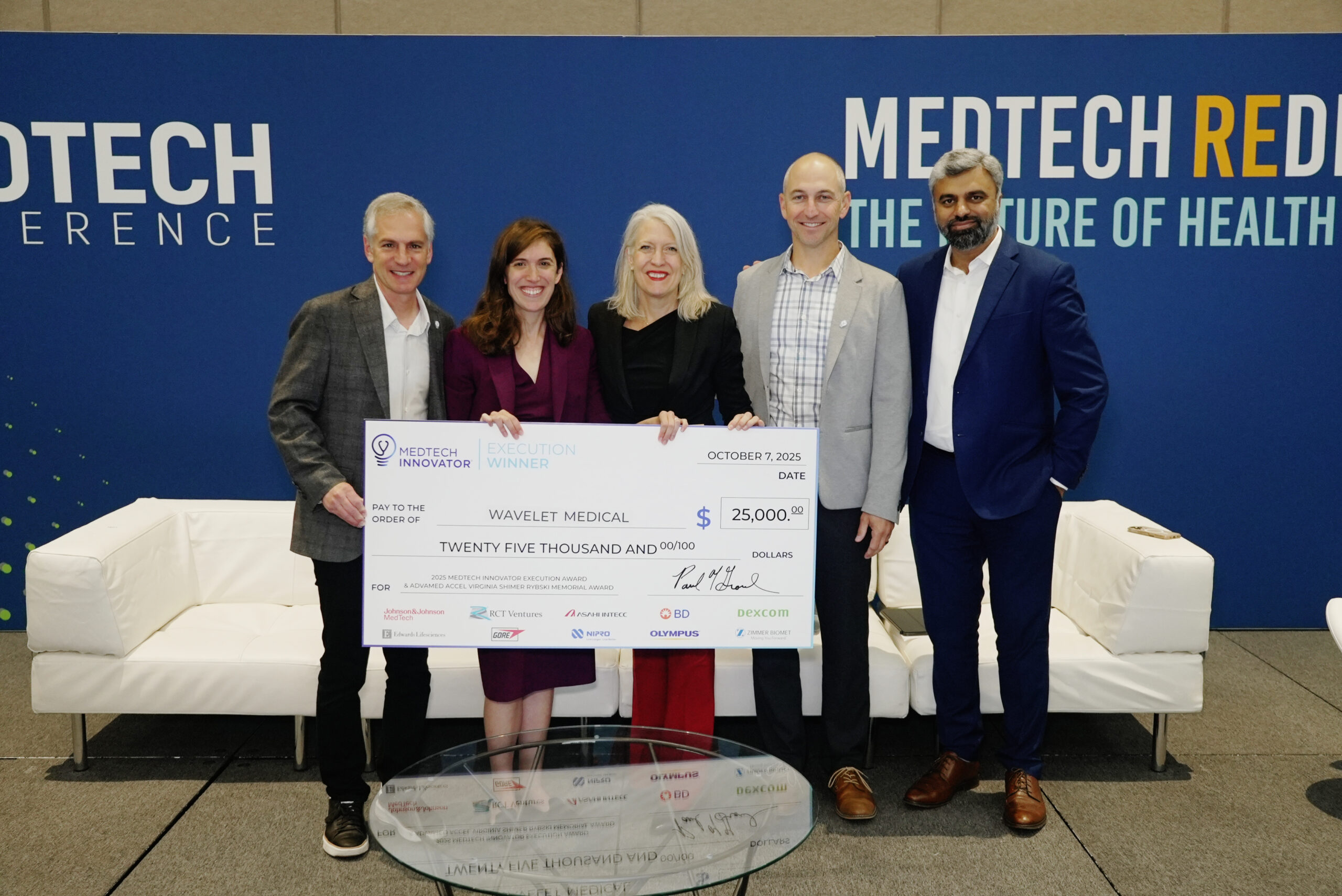
MedTech Innovator is the world's largest accelerator for medical device, digital health, and diagnostic companies. Our mission is to improve the lives of patients by accelerating the growth of companies that are transforming the healthcare system.
Introducing the Innovator Job Board
As the 700+ companies in our ecosystem grow, they’re looking for exceptional talent to help them. Our Innovator Job Board highlights hundreds of opportunities in every possible discipline a growing company needs to thrive. If you’re looking for your next great opportunity to improve healthcare, check out the listings here!

Press Releases and Announcements
MedTech Innovator Announces 2025 Mid-Stage Prize and Value Award Finalists
Portfolio Company News
DeviceTalks Weekly podcast: Armor Medical’s wearable could give earlier warnings of severe hemorrhaging before it’s too late
Closing the Patient Support Gap: How Ankr Health’s 4X Platform Transforms Oncology Care
Explore MedTech Innovator’s Portfolio

Enabling the Development of Innovations in Drug Discovery and Development and Biomanufacturing to Improve Clinical Research and Care Coordination
BioTools Innovator VANGUARD is at the forefront of biomanufacturing innovation, enabling life science tools startups to develop solutions that improve drug discovery and development; increase speed, capacity, portability, and scalability; and improve clinical research and clinical care coordination. VANGUARD stands ready to support startups in deploying innovative medical countermeasures that enhance the United States’ readiness for public health emergencies.
BioTools Innovator VANGUARD consists of two programs, a wraparound Accelerator program as part of the core BioTools Innovator annual cohort, and a specialized product Development, Evaluation, and Validation (DEV) funding opportunity. Companies may apply to either or both programs.
Learn more at biotoolsinnovator.org/vanguard